The pharmacology of INGREZZA® (valbenazine) capsules
>>The pharmacology of INGREZZA® (valbenazine) capsules
[On-screen text reads, “The pharmacology of INGREZZA (valbenazine) capsules.”]
>>Important Information
>>INDICATION & USAGE: INGREZZA® (valbenazine) capsules and INGREZZA® SPRINKLE (valbenazine) capsules are indicated in adults for the treatment of tardive dyskinesia and for the treatment of chorea associated with Huntington’s disease.
>>IMPORTANT SAFETY INFORMATION
>>Depression and Suicidality in Patients with Huntington’s Disease: VMAT2 inhibitors, including INGREZZA and INGREZZA SPRINKLE, can increase the risk of depression and suicidal thoughts and behavior (suicidality) in patients with Huntington’s disease. Balance the risks of depression and suicidality with the clinical need for treatment of chorea. Closely monitor patients for the emergence or worsening of depression, suicidal ideation, or unusual changes in behavior. Inform patients, their caregivers, and families of the risk of depression and suicidal ideation and behavior and instruct them to report behaviors of concern promptly to the treating physician. Exercise caution when treating patients with a history of depression or prior suicide attempts or ideation, which are increased in frequency in patients with Huntington’s disease.
>>Please see additional Important Safety information at the end of this video.
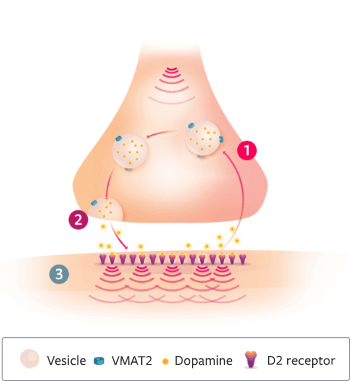
>>VMAT2 is a proven target when it comes to treating tardive dyskinesia and Huntington’s disease chorea.
[Animation of a target slides into frame. The target reads, “VMAT2.” Footnote at the bottom of the screen reads, “The mechanism of action of INGREZZA is unclear, but is believed to be mediated through the selective inhibition of VMAT2.”]
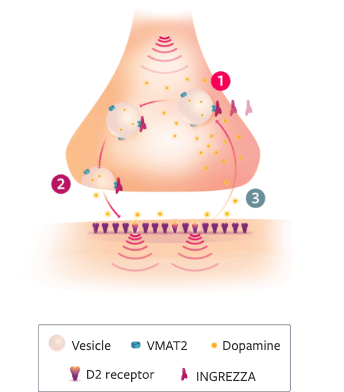
>>And INGREZZA is a treatment with a precise focus on VMAT2.
[The INGREZZA logo appears on the screen.]
>>As a uniquely selective treatment…
[The INGREZZA logo fades away and transitions to become the tip of an arrow placed within a bow. Text next to bow and arrow reads, “Uniquely selective.” Footnote at the bottom of the screen reads, “Based on in vitro data. The clinical significance of in vitro data is unknown and is not meant to imply clinical outcomes.”]
>>…INGREZZA is metabolized into a single primary metabolite, + alpha.
[Animation zooms in on pink arrowhead, labeled with a + alpha symbol.]
>>The + alpha metabolite has high affinity and selectivity for VMAT2 with no appreciable off-target affinity.
[Animation of arrow launches across the frame. Footnote at the bottom of the screen reads, “Based on in vitro VMAT2 binding affinity of dihydrotetrabenazine (HTBZ) metabolites and INGREZZA’s primary active metabolite, + alpha HTBZ. The clinical significance of in vitro data is unknown and is not meant to imply clinical outcomes.”]
>>With 100% aimed at VMAT2...
[Multiple INGREZZA arrows hit the target’s bullseye labeled, “VMAT2.”]
>>...INGREZZA is targeted where it matters.
[The INGREZZA arrows remain on the VMAT2 target.]
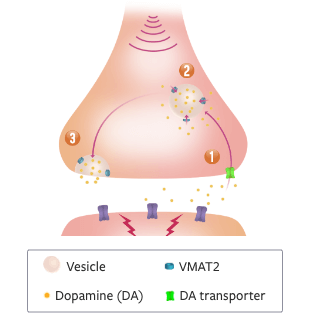
>>Other VMAT2 inhibitors…
[Two quivers filled with arrows appear on screen. One is labeled “Tetrabenazine,” and the other is labeled “Deutetrabenazine.”]
>>…are converted into 4 isomers.
[Animation focuses in on the deutetrabenazine-filled quiver. Four arrows lift out of the quiver.]
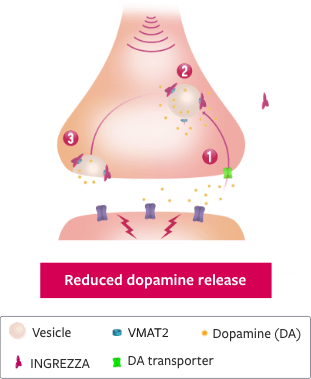
>>The most abundant isomer of deutetrabenazine has appreciable affinity for off-target receptors and negligible affinity for VMAT2.
[The isomer arrows pull completely out of the quiver, which leaves the frame. Once out of the quiver, the four arrows stack up vertically. The top arrow is orange and labeled – alpha followed by 66%; the second is purple and labeled + beta followed by 29%; the third arrow is blue color and labeled – beta followed by 3%; the fourth arrow is red and labeled + alpha followed by 2%.]
[Animation of the four different colored arrows fly across the screen. The number of each type of arrow is in relative proportion to the percentages labeled in the previous screen.]
[All arrows fly toward a target labeled VMAT2, but most do not hit the target—only one red arrow and three purple arrows. Footnote at the bottom of the screen reads, “The activity of these compounds is based on in vitro data and the clinical implications are unknown. These data do not imply superiority of any compound. Head-to-head trials comparing deutetrabenazine or tetrabenazine to valbenazine have not been conducted.”]
>>When all you want is VMAT2 inhibition…
[A large group of arrows representing INGREZZA fly across the screen.]
>>…all you need is INGREZZA.
[INGREZZA arrows all hit the VMAT2 target bullseye.]
>>Only INGREZZA is uniquely selective for VMAT2.
>>[“INGREZZA” jingle]
[The INGREZZA logo is centered on screen.]
>>Important Information
>>INDICATION & USAGE: INGREZZA® (valbenazine) capsules and INGREZZA® SPRINKLE (valbenazine) capsules are indicated in adults for the treatment of tardive dyskinesia and for the treatment of chorea associated with Huntington’s disease.
>>IMPORTANT SAFETY INFORMATION
>>Depression and Suicidality in Patients with Huntington’s Disease: VMAT2 inhibitors, including INGREZZA and INGREZZA SPRINKLE, can increase the risk of depression and suicidal thoughts and behavior (suicidality) in patients with Huntington’s disease. Balance the risks of depression and suicidality with the clinical need for treatment of chorea. Closely monitor patients for the emergence or worsening of depression, suicidal ideation, or unusual changes in behavior. Inform patients, their caregivers, and families of the risk of depression and suicidal ideation and behavior and instruct them to report behaviors of concern promptly to the treating physician. Exercise caution when treating patients with a history of depression or prior suicide attempts or ideation, which are increased in frequency in patients with Huntington’s disease.
>>CONTRAINDICATIONS: INGREZZA and INGREZZA SPRINKLE are contraindicated in patients with a history of hypersensitivity to valbenazine or any components of INGREZZA or INGREZZA SPRINKLE.
>>WARNINGS AND PRECAUTIONS
>> Hypersensitivity Reactions: Hypersensitivity reactions, including cases of angioedema involving the larynx, glottis, lips, and eyelids, have been reported in patients after taking the first or subsequent doses of INGREZZA. Angioedema associated with laryngeal edema can be fatal. If any of these reactions occur, discontinue INGREZZA or INGREZZA SPRINKLE.
>>Somnolence and Sedation: INGREZZA and INGREZZA SPRINKLE can cause somnolence and sedation. Patients should not perform activities requiring mental alertness such as operating a motor vehicle or operating hazardous machinery until they know how they will be affected by INGREZZA or INGREZZA SPRINKLE.
>>QT Prolongation: INGREZZA and INGREZZA SPRINKLE may prolong the QT interval, although the degree of QT prolongation is not clinically significant at concentrations expected with recommended dosing. INGREZZA and INGREZZA SPRINKLE should be avoided in patients with congenital long QT syndrome or with arrhythmias associated with a prolonged QT interval. For patients at increased risk of a prolonged QT interval, assess the QT interval before increasing the dosage.
>> Neuroleptic Malignant Syndrome: A potentially fatal symptom complex referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with drugs that reduce dopaminergic transmission, including INGREZZA. The management of NMS should include immediate discontinuation of INGREZZA or INGREZZA SPRINKLE, intensive symptomatic treatment and medical monitoring, and treatment of any concomitant serious medical problems. If treatment with INGREZZA or INGREZZA SPRINKLE is needed after recovery from NMS, patients should be monitored for signs of recurrence.
>>Parkinsonism: INGREZZA and INGREZZA SPRINKLE may cause parkinsonism. Parkinsonism has also been observed with other VMAT2 inhibitors. Reduce the dose or discontinue INGREZZA or INGREZZA SPRINKLE treatment in patients who develop clinically significant parkinson-like signs or symptoms.
>>ADVERSE REACTIONS: The most common adverse reaction in patients with tardive dyskinesia (≥5% and twice the rate of placebo) is somnolence. The most common adverse reactions in patients with chorea associated with Huntington’s disease (≥5% and twice the rate of placebo) are somnolence/lethargy/sedation, urticaria, rash, and insomnia.
>>You are encouraged to report negative side effects of prescription drugs to the FDA. Visit MedWatch at www.fda.gov/medwatch or call 1-800-FDA-1088.
>>Dosage Forms and Strengths: INGREZZA and INGREZZA SPRINKLE are available in 40 mg, 60 mg, and 80 mg capsules.
>>Please see full Prescribing Information, including Boxed Warning, at INGREZZAHCP.com.
[Blue gradient builds into screen with logos, references, and codeline.]
[On-screen text reads, “REFERENCES: 1. Grigoriadis DE, Smith E, Hoare SRJ, Madan A, Bozigian H. Pharmacologic characterization of valbenazine (NBI-98854) and its metabolites. J Pharmacol Exp Ther. 2017;361(3):454-461. 2. Brar S, Vijan A, Scott FL, et al. Pharmacokinetic and pharmacologic characterization of the dihydrotetrabenazine isomers of deutetrabenazine and valbenazine [published online ahead of print, 2022 Dec 18]. Clin Pharmacol Drug Dev. 2022;10.1002/cpdd.1205. 3. Skor H, Smith EB, Loewen G, O’Brien CF, Grigoriadis DE, Bozigian H. Differences in dihydrotetrabenazine isomer concentrations following administration of tetrabenazine and valbenazine. Drugs R D. 2017;17(3):449-459. 4. Data on file. Neurocrine Biosciences, Inc.”]
©2024 Neurocrine Biosciences, Inc. All Rights Reserved. EP-VBZ-US-0039 09/2024